Autologous Platelet Gel and PRF in Implant Dentistry

INTRODUCTION
Bone and soft-tissue deficiency before implant placement can be a new challenge to many clinicians; extensive bone- and soft-tissue grafting remains a delicate procedure, because of the slow and difficult integration of the grafted material into the physiological architecture. Materials and techniques have been developed to overcome these deficiencies and have shown promising outcomes. However, complications associated with grafting procedures do occur. These complications challenge clinicians ability to: identify etiologies; provide proper, timely management; and, hopefully, institute protocols to minimize occurrences in the future.
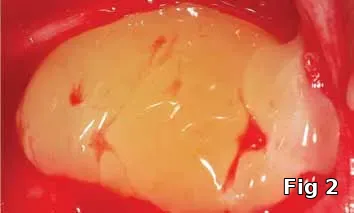
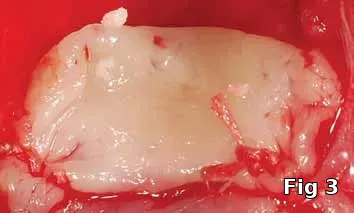
The purpose of this article is to introduce the preparation and intra-operative use of platelet gel (Fig. 1), an autologous formulation of platelet-rich fibrin (PRF) membrane (Fig. 2) and gel (Fig. 3) for the purpose of preventing complications resulting from incomplete wound closure at the time of surgery.
Bone grafting has become an essential part of implant dentistry. Nonetheless, the complications associated with bone-grafting procedures have slowly emerged to be one of the main challenges to many clinicians. Implant-associated bone-grafting procedures are becoming more and more popular and standard of care, but complications related to bone grafting are several and their prevention is of the utmost importance.¹
Use of membrane for guided bone regeneration (GBR) to contain and allow for the environment necessary for graft maturation is paramount. The use of membrane for GBR also runs a risk of flap sloughing, more with non-resorbable membranes than with resorbable and exposure not only of the membrane but also of the micro screws holding it into place, leading to bacterial contamination and infection.² Without complete soft-tissue coverage over the socket or complete seal of the sinus membrane perforation, contamination or loss of graft materials placed can also occur. This can lead to premature membrane and graft removal.³,⁴
Tension-free flap closure is essential so exposure of membrane or fixation screws can be prevented.⁵ The development of a tension-free flap for an advancement type primary closure is not without its challenges. Anatomic considerations as in the mandibular premolar area due to the presence of the mental foramen and neuro-vascular bundle (NVB), esthetic considerations as in the anterior maxilla, existing inadequate soft-tissue architecture either from recession and/or thin biotype, might not allow for split thickness flap development or lead to inadvertent perforation of the gingiva or mucosa can compromise the surgical results.⁶
Reduction of keratinized tissue at the facial aspect with primary closure may also be a potential complication. However, this can be avoided by allowing the socket to heal for six to eight weeks before grafting. Newly formed keratinized tissue growing over the socket will then provide necessary primary closure without sacrificing the facial width of keratinized tissue. This approach to manage the inadequate amount of keratinized tissue delays the planned prosthetic rehabilitation by the said time.",
Use of platelet concentrates, like platelet rich plasma (PRP), mixed into the hard-tissue replacement graft and PRF for mucosal healing aims to improve this process of integration by accelerating bone and mucosal healing. PRF is a healing biomaterial that concentrates in a single autologous fibrin membrane, most platelets, leukocytes and cytokines from blood harvest at the time of surgery, without artificial biochemical modification (no anticoagulant and no bovine thrombin) and without the need for predonation and costly processing or the use of homologous blood products.
Whether used as a membrane or as fragments, PRF allows a significant postoperative protection of the surgical site and seems to accelerate the integration, remodeling and healing of the soft tissue and it provides a very high quality of gingival maturation. This fibrin biomaterial represents a new opportunity to improve both the maturation and the final esthetic result of the peri-implant soft tissue. This innovative biomaterial can be defined as a platelet and immune concentrate⁷⁻¹⁰ that combines in a single fibrin membrane most healing- and immunity-conducive constituents from a sample of blood.
Unlike other platelet concentrates,¹¹⁻¹³ this technique requires neither anticoagulants nor bovine thrombin. It is nothing other than centrifuged blood without additives. The protocol is very simple: Whole blood is drawn in 10-mL tubes without anticoagulant and is immediately centrifuged at around 400 g and 2700 rpm for 12 minutes. Within a few minutes, the absence of anticoagulant allows the activation of the majority of the platelets contained in the sample, in contact with the tube walls, thus triggering a coagulation cascade.
],
The fibrinogen is at first concentrated in the upper part of the tube, until the effect of the circulating thrombin transforms it into a fibrin network. The result is a fibrin clot located in the middle of the tube and soaked with acellular plasma, with a maximum number of platelets caught in the fibrin mesh. When used as a membrane, PRF enables the protection of operative sites from outside aggression and serves as a matrix to accelerate the healing of wound edges,¹⁴ much like a fibrin bandage.¹⁰,¹⁵
When mixed with the graft material, the fibrin clot functions as a biological connector between the different elements of the graft, and as a matrix which favors neo-fibrinogenesis, neo-angiogenesis, and promotion of cellular differentiation, and acceleration of the effects of growth factors on other cells, such as macrophages. The capture of stem cells, and the liquid medium found within the gel, causes migration of osteoprogenitor cells to the center of the bone graft.⁹,¹⁶
Wetting the surface with platelet derived growth factors (PRGF) has given rise to the first bioactive surface, the use of which significantly speeds up the healing process. There is already ample evidence that growth factors play a crucial role in the process of healing and tissue regeneration. As the role of these growth factors in tissue regeneration is gradually better understood, the conclusion has at least been reached that their release, and therefore their controlled expression in the area of tissue damage or injury, could be fundamental in the treatment of injuries.
Preparations rich in growth factors also release bioactive proteins at the localized site, with the aim of triggering healing and regenerative processes. It has been observed and reported that soft-tissue healing occurs at an accelerated rate and results in keratinized tissue formation. The molecular pool results from the release by PRGF scaffolds influence cell proliferation, release of angiogenic growth factors (VEGF, HGF), synthesis of type 1 collagen and hyaluronic acid (HA) and inflammation.
HA is naturally found in the extracellular space and acts as an intercellular binding agent and can bind up to 10 times its weight to water. Platelets help to prevent blood loss at sites of vascular injury. To do this, they adhere aggregate and form a procoagulant and fibrin formation. In addition, platelets express and release substances that promote tissue repair and influence processes such as angiogenesis, inflammation and the immune response.
They contain a secretable pool of large number of biologically active proteins while newly synthesized active metabolites are also released. Although anucleated platelets possess spliceosomes and can synthesize tissue factors and interleukin 1b. The binding of secreted proteins within a developing fibrin mesh or to the extracellular matrix can create chemotactic gradients accelerating cell migration and differentiation as well as recruitment of stem cells.
This administration in a fibrin glue provides an adhesive support that helps confine accelerated biologic activity to a chosen site. In a comparative study comparing the proliferation and synthesis of type 1 collagen and angiogenic factors in human tenocytes cultured in a PGRF matrix and platelet poor matrix. The results show that the PRGF induces greater cellular proliferation and a better synthesis of VEGF (the principle angiogenic factor).
The cell cultures stimulated with PRGF secreted HGF (anti fibrotic factor) and some type 1 collagen. In a split mouth study evaluating the impact of plasma rich in growth factors on clinical and biologic factors involved in healing processes after third molar extractions, it was found that in all the cases, postoperative pain and swelling, measured at all experimental times, were reduced in the presence of PRGF. The clinical cases that follow are a detailed illustration of this concept.
CLINICAL ILLUSTRATION
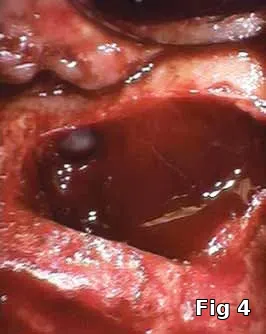
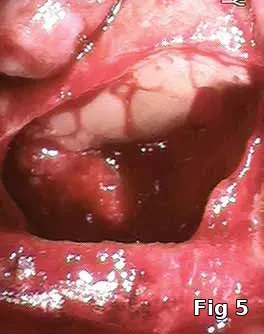
Sinus membrane perforation repair: Even with the best surgical technique, perforations in the sinus membrane can occur (Fig. 4). Most are small enough to not cause migration of the graft material into the sinus, but it has been suggested that even those small perforations, not to mention large ones should be sealed (Fig. 5) prior to introduction of the bone-graft material to avoid graft migration and bacterial contamination of the graft from the normal sinus flora.
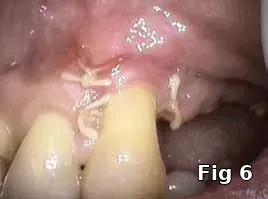
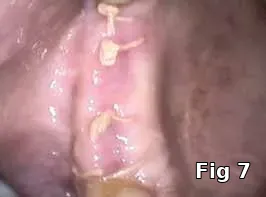
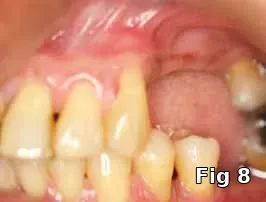
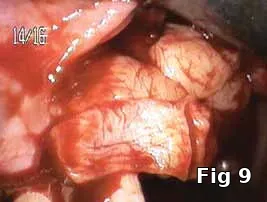
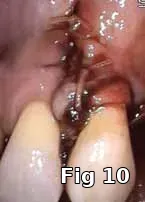
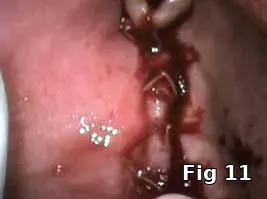
Flap closure management (Figs. 6–11): This patient is a 68-year-old female who has poorly controlled Type 2 Diabetis Melitus (DM) and is a one-pack-a-day smoker and refuses to not wear her partial denture during the primary soft-tissue healing phase (Fig. 6). In cases of possible delayed or compromised healing, using concentrated multifactorial growth factors at the site of injury (Fig. 7) prior to closure (Figs. 8–9) decreases those possibilities and improves and accelerates soft-tissue healing (Figs. 10–11) and diminishes the pain patients feel because of post operative inflammation.
Extraction socket soft-tissue closure: This patient is a 90-year-old male with Type 2 DM (poorly controlled), hypertension, congestive heart failure, osteoporosis and anticoagulant therapy with strict instructions from cardiologist to not discontinue his anticoagulant therapy and is classified as ASA3, presented for the extraction of severely decayed and periodontally involved non-salvageable tooth #30. Among other things, he has poor oral hygiene and is noncompliant with dental care recommendations.
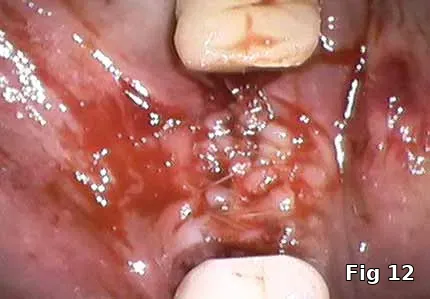
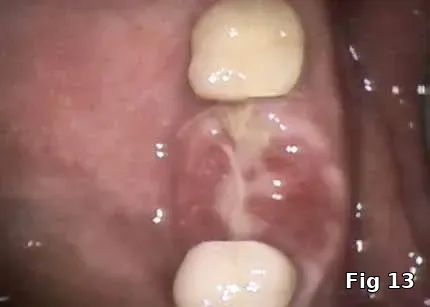
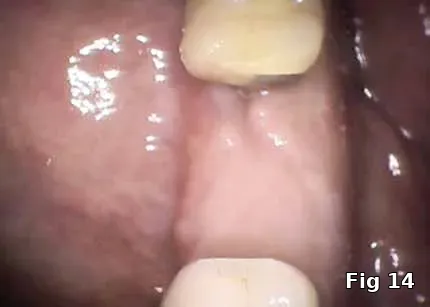
Healing and postoperative infections are a major concern with him as he has persistent non-healing decubitus ulcers and had his right foot amputated as a complication of his DM. Here, the PRGF graft was extremely helpful as it brought to the area concentrated volume of platelets even with the anticoagulant therapy on board for immediate hemostasis (Fig. 12). Healing cascade was enhanced and accelerated as well was the built-in antiinflammatory component (Fig. 13, one week postoperative view and Fig.14, two months postoperative view).
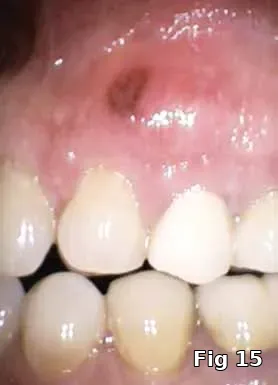
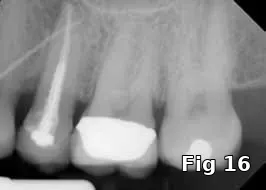
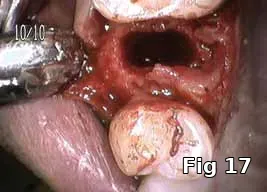
Ridge preservation — use as a membrane: Here is a 40-year-old male who presented for the evaluation of a painful swelling in his gum (Fig. 15). Diagnosis was peri-apical abscess with sinus fistula, possible midroot fracture of tooth #13 (Fig. 16) and a treatment plan to extract the tooth (Fig. 17) and ridge preserve followed by implant placement and an implant-supported fixed restoration.
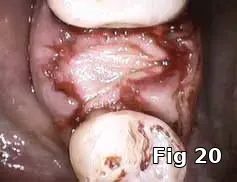
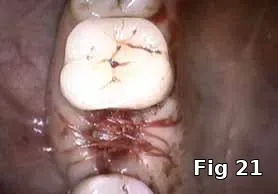
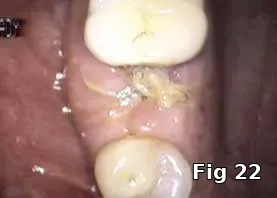
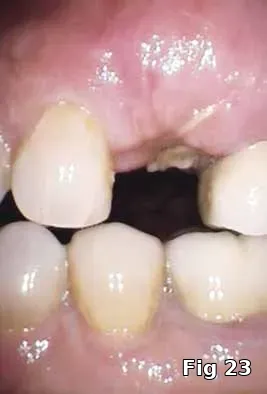
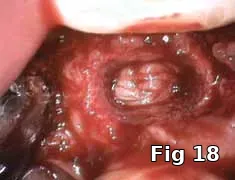
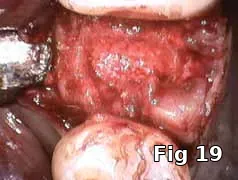
Here PRGF graft was helpful in securing the buccal fenestration defect at the apex prior to bone grafting (Fig. 18) allowing for secure placement and containment of the bone graft (Fig. 19) and PRGF membrane (Fig. 20) for ridge preservation. Flaps were readapted and closed over the PRGF membrane (Fig. 21) and one-week postoperative shows complete closure of the soft tissue over the extraction socket (Fig. 22) and complete resolution of the sinus tract and swelling in the buccal gingival (Fig. 23).
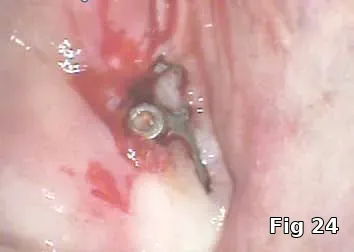
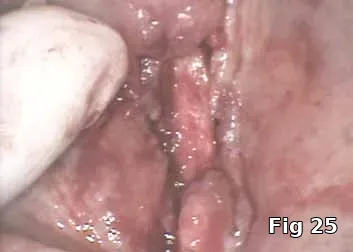
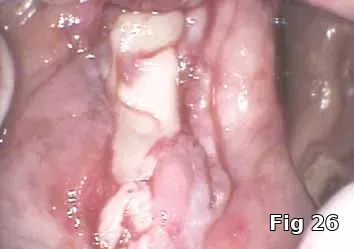
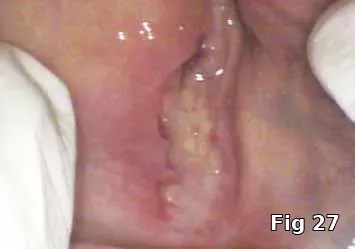
Regeneration of dehiscence: Long-term successful maintenance of soft tissue around implants can be challenging. In this case of dehiscence of the soft tissue and chronic inflammation around a subperiosteal implant (Fig. 24), the exposed implant strut was sectioned (Fig. 25) and removed, and PRGF grafts were used to fill in the defect left behind (Fig. 26) and accelerate healing and granulation of the soft tissues (Fig. 27).
MEMBRANE FOR (GBR) POST APICOECTOMY
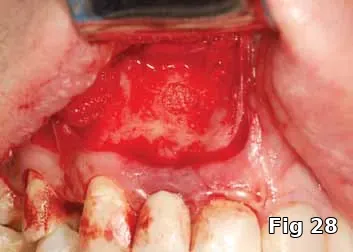
Here is a 24-year-old male with an ASA1 medical status who presented for the treatment of an apical lesion prior to prosthetic replacement of the failing existing restoration secondary to recurrent decay. Treatment plan to perform an apicoectomy and retrofill with MTA with Beta phase Tri Calcium Phosphate (B-TCP) bone graft saturated with PRP for GBR (Fig. 28).
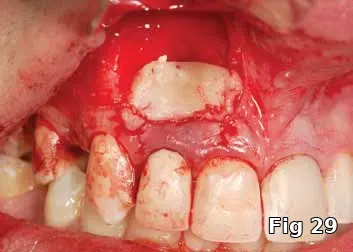
PGRF fibrin membrane was developed and secured into place by resorbable sutures (Fig. 29).
DISCUSSION
Autologous platelet gel has been used with apparent clinical success in a variety of applications in many fields of medicine. In our practice, we have successfully used this technique in conjunction with reconstructive surgical procedures of the maxillo-mandibular reconstruction, surgical repair of alveolar and gingival defects, oral-antral fistulas and perforations as a result of extractions or sinus lift, and in adjunctive procedures related to the placement and management of osseointegrated and subperiosteal implants.
We routinely use autologous fibrin mesh during alveolar reconstruction and socket preservation as our preferred reconstructive approach for osseous stabilization and soft-tissue reconstruction at the time of extraction. This allows for complete soft-tissue closure and healing over the collagen membrane when there is <10 mm gap in the flap closure. As the soft tissues are closed in the regular fashion, a thick layer of platelet gel is applied to the underlying tissues.
The adhesive nature of the fibrin gel enables predictable flap adaptation and hemostasis. As the gel consolidates, a more definitive seal is obtained than with primary closure alone. Even with meticulous handling of the soft tissues, it is often difficult to obtain an ideal approximation and watertight closure. The gel is applied to the area of tissue approximation to provide a seal over the membrane.
Use of the PRF in conjunction with maxillary sinus lift procedure has been similarly successful in both cases of perforation and no observable perforation of the Schneiderian membrane. When an observable perforation occurs, the self-sealing properties of the fibrin gel come in extremely handy as the gel literally sticks to the membrane and restores the membrane function, immediately allowing for the consolidation of the bone graft.
Even when there is no observable perforation, in cases of severe pneumatizations, by placing the gel against the lining of the membrane, the bone graft can be compressed against it without fear of tear. PRF additionally comes in extremely handy when placed under the incisions prior to closure or to fill any voids left after closure by simply tucking the gel under the margins of the closure.
In cases in which an inferior alveolar nerve (IAN) lateralization procedure is required, the platelet gel provides consolidation of the bone fragments and packed lateral to the nerve wrapped in the fibrin mesh after implant placement isolating the nerve from direct contact with both the particulate graft and implants. The fibrin mesh under the flap allows for faster healing and reduced chances of postoperative complications.
CONCLUSION
From this study, it can be concluded that:
- The use of PRF and PRP is one strategy that can modulate and enhance wound healing by sequestering and concentrating the platelets > 300 percent more and therefore the many growth factors they contain. These growth factors are the universal initiators of almost all wound healing. By taking advantage of all of the natural regenerative pathways, and using all of the known and yet to be identified growth factors in platelets, autologous platelet derived growth factors, which are nontoxic and nonimmunoreactive, accelerate existing wound healing pathways.
- Platelet rich fibrin and plasma also modulate and upregulate one growth factor’s function in the presence of second or third growth factor. It is this specific feature that separates platelet rich growth factors from recombinant growth factors, which are single growth factors that focus on only one regenerative pathway. Recombinant growth factors, therefore, may not be as functional in wounds because of rate limitations from nonenhanced levels of concomitant regulating growth factors.
- The presence of concentrated platelets in the formulation brings cytokines and growth factors to the site of surgery that normally would not occur normally.
REFERENCES
- Jingjing Li, Wang HL. Common implant-related advanced bone grafting complicatons: classification, etiology, and management. Implant Dentistry: 2008;17:389-401.
- Schlegel KA, Sindet-Pedersen S, Hoepffner HJ. Clinical and histological findings in guided bone regeneration (GBR) around titanium dental implants with autogenous bone chips using a new resorbable membrane. J Biomed Mater Res. 2000;53:392-399.
- Froum S, Cho SC, Elian N, et al. Extraction sockets and implantation of hyroxyapatities with membrane barriers: A histologic study. Implant Dent. 2004;13:153-164.
- Block MS, Kent JN. Sinus augmentation for dental implants: The use of autogenous bone. J Oral Maxillofac Surg. 1997;55:1281-1286.
- del Castillo RA. Defect morphology: Effects on regenerative predictability and membrane selection. Compend Contin Educ Dent. 2000;21:383-385, 388, 390.
- Carlson ER, Monteleone K. An analysis of inadvertent perforations of mucosa and skin concurrent with mandibular reconstruction. J Oral Maxillofac Surg. 2004;62:1103- 1107.
- Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. I. Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37-e44.
- Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. II. Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45-e50.
- Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. III. Leucocyte activation: A new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e51-e55.
- Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. IV. Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56-e60.
- Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638-646.
- Weibrich G, Kleis WK, Buch R, et al. The Harvest Smart PRePTM system versus the Friadent-Schutze platelet-rich plasma kit. Clin Oral Implants Res. 2003;14:233-239.
- Dohan DM, Del Corso M, Charrier JB. Cytotoxicity analyses of Choukroun`s platelet-rich fibrin (PRF) on a wide range of human cells: The answer to a commercial controversy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:587-593.
- Choukroun J, Braccini F, Diss A, et al. Influence of platelet rich fibrin (PRF) on proliferation of human preadipocytes and tympanic keratinocytes: A new opportunity in facial lipostructure (Coleman`s technique) and tympanoplasty? Rev Laryngol Otol Rhinol (Bord). 2007;128:27-32.
- Charrier JB, Steve M, Albert S, et al. Relevance of Choukroun`s platelet-rich fibrin (PRF) and SMAS flap in primary reconstruction after superficial or subtotal parotidectomy in patients with focal pleiomorphic adenoma: A new technique. Rev Laryngol Otol Rhinol (Bord). In press.
- Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. V. Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299-303.